“Vertex’s blockbuster-in-waiting painkiller suzetrigine set for FDA review next year” … “Vertex posts full pain data ahead of FDA ruling, unpacking combo results, Vicodin miss and stellar safety” … What’s behind these headlines? Let’s break down the buzz around Vertex, a company we valued earlier this year, and explore what’s driving its momentum. [ TL;DR at the end of the page ]. Vertex Pharmaceutical Inc., VRTX, is a global biotech company that create and sell transformative medicines for people with serious diseases. In particular the company has approved medicines that treat the underlying causes of multiple chronic, life-shortening genetic diseases — cystic fibrosis, sickle cell disease and transfusion-dependent beta thalassemia. Plus several clinical and research programs are underway, including acute and neuropathic pain, APOL1-mediated kidney disease, IgA nephropathy, autosomal dominant polycystic kidney disease, type 1 diabetes, myotonic dystrophy type 1 and alpha-1 antitrypsin deficiency. Vertex is a multinational company, founded in 1989, global headquarters in Boston, with international headquarters in London. It has research and development sites and commercial offices in North America, Europe, Australia, Latin America and the Middle East. From a governance and social point of view, Vertex is consistently recognized as one of the industry's top places to work, including 14 consecutive years on Science magazine's Top Employers list and one of Fortune’s 100 Best Companies to Work For. On Jul. 30, 2024 VRTX announced that: “the U.S. Food and Drug Administration (FDA) has accepted its New Drug Application (NDA) submission for suzetrigine, an investigational, oral, selective NaV1.8 pain signal inhibitor to treat moderate-to-severe acute pain. Suzetrigine has the potential to be the first new class of medicine to treat acute pain in over twenty years. The FDA has granted suzetrigine priority review and assigned a Prescription Drug User Fee Act (PDUFA) target action date of January 30, 2025. Suzetrigine has already been granted FDA Fast Track and Breakthrough Therapy designations for the treatment of moderate-to-severe acute pain. “ Now let’s break this down. The premises seam great, but is gold that glitters? The reader can skip this part if in a rush, it does not add to the financial picture of VRTX. As you may already know, every the pharma industry plays a vital role in modern healthcare, focusing on developing medications to improve and save lives. It is driven by innovation and substantial investment in research, particularly in discovering new treatments and conducting clinical trials. In the United States, the Food and Drug Administration (FDA) oversees the drug approval process to ensure treatments meet strict safety and effectiveness standards. This multi-step process begins with preclinical testing in labs, followed by three phases of clinical trials in humans. If the trials demonstrate that a drug is safe and effective, the company submits a New Drug Application (NDA) or Biologics License Application (BLA) for FDA review. After approval, the drug enters the market under continued post-marketing surveillance to monitor its safety and impact on public health. So, prior to approval, each drug marketed in the United States must go through a detailed FDA review process. In 1992, under the Prescription Drug User Fee Act (PDUFA), FDA agreed to specific goals for improving the drug review time and created a two-tiered system of review times – Standard Review and Priority Review. A Priority Review designation means FDA’s goal is to take action on an application within 6 months (compared to 10 months under Standard Review). Short answer: no. They pay for FDA staff members to quickly review the application but still take decisions based on solid scientific evidence. The Prescription Drug User Fee Act (PDUFA) was created by Congress in 1992 and authorizes FDA to collect user fees from persons (pharma companies) that submit certain human drug applications for review or that are named in approved applications as the sponsor of certain prescription drug products. For pharmaceutical companies hoping to win regulatory approval for a drug, time really does mean money. The longer it takes for the U.S. Food and Drug Administration (FDA) to make an approval decision, the longer it is before a drugmaker can begin making money to recover the cost of its investment to develop the therapy. These costs vary significantly depending on therapeutic areas and other factors, with some studies reporting a range from $314 million to $4.46 billion - oncology compounds being the most expensive. However, a broader consensus highlights the $2 billion range as a representative average for successfully approved drugs. The PDUFA's primary goal was to authorize the FDA to collect fees from drugmakers to help pay for the FDA staff needed to review regulatory filings for drugs. The PDUFA helps the FDA by providing the agency with a way to generate money and establishes a set timeline for the agency to make approval decisions. Since the enactment of PDUFA in 1992, FDA's spending from “user fees” has generally increased, both in absolute terms and as a share of FDA's total budget, accounting for over 45% of the agency's FY2020 total program level. The remaining part being founded by the Congress.Vertex, the Company
The News, Incoming Action Date for an Acute Pain non-opioid Medication
FDA Process - PDUFA - FDA Budget
The FDA Granted VRTX Priority Review
Why “User Fee” in the PDUFA? Does companies pay for a definite action date?
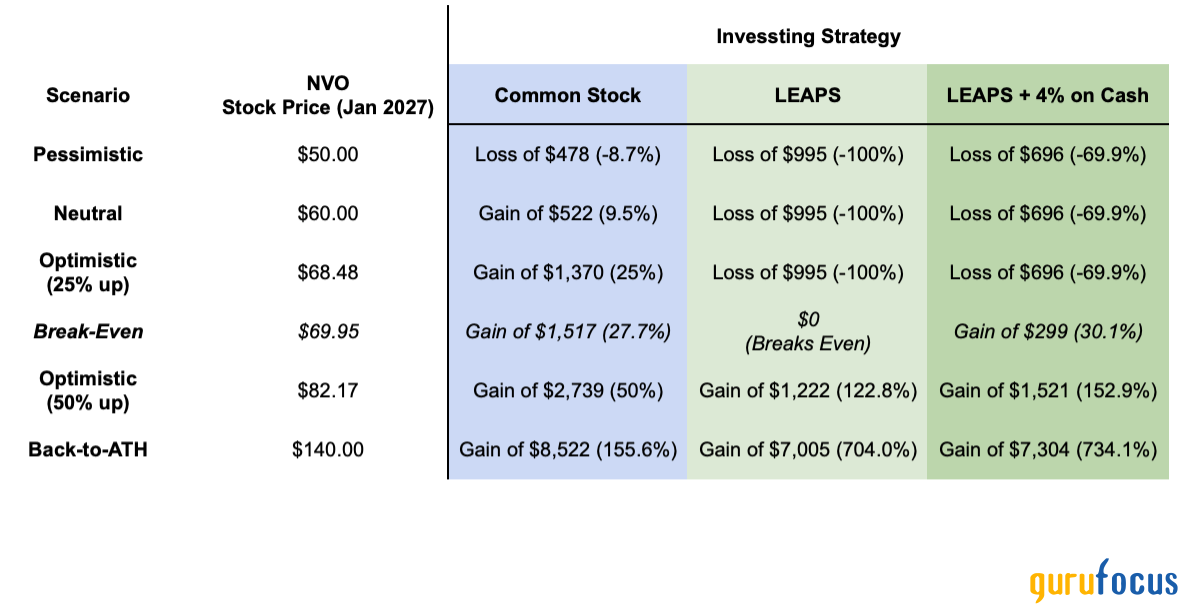
Images/tables from: Short answer: It is an indicative date by which the FDA commit to formalizing a decision. The date is not set in stone. The FDA does occasionally extend the review period, most commonly when additional data is required from the drugmaker or the company itself submits additional data that requires more time to review. Prior to 1992, the FDA didn't have to make approval decisions within a specified period. This caused frustration for drugmakers, along with physicians and patients hoping for new treatments. The FDA doesn't publish an official list containing all of the outstanding PDUFA dates for drugs under review. However, many drugmakers announce when they receive a PDUFA date from the agency for one of their drugs. Once the FDA accepts a filing for the approval of a drug, the agency must complete its review process within 10 months in most cases. In some instances, the FDA grants Priority Review status to the regulatory filing for a drug on the base of sound clinical and societal implications (the PDUFA data is 6 months from the acceptance of the submission in this case). Out of curiosity, these are the fees requested by the FDA for the various applications and program. The base revenue (expected revenue) amount for FY 2025 is $1,358,764,346. Fee amounts are to be established each year so that revenues from application fees provide 20% of the total revenue, and prescription drug program fees provide 80%. Third Quarter 2024 Results were encouraging. A 12% increase in product revenue (to $2.77 billion) with a full-year product revenue guidance raised to $10.8 billion to $10.9 billion. The growth was evenly spread in the U.S. (+10%, to $1.71 billion) and outside the US (+14%, to $1.06 billion), compared to the third quarter of 2023. Acquired IPR&D (AIPR&D) expenses were $15 million compared to $52 million in the third quarter of 2023. Combined R&D and SG&A expenses were in line with previous year (a little bit more than $1 billion each), with the small increases linked to commercial investment to support launches of Vertex's therapies globally and continued investment in support of additional programs that have advanced to Phase 3 clinical development. GAAP Net income was $1.0 billion. A stable figure due to the offset of increased product revenue with increased R&D and SG&A expenses, compared to the Q3-23. Cash, cash equivalents and total marketable securities as of September 30, 2024 were $11.2 billion, still abundant. All data are from the official VRTX source. We don’t think so. Remember that approximately $4.9 billion in cash ($4.6 billion net of estimated cash acquired) was paid to acquire Alpine Immune Sciences in Q2-2024. The move was unanimously approved by both the Vertex and Alpine Boards of Directors. Alpine is a clinical stage biotech company focused on protein-based immunotherapies. The lead product, povetacicept, demonstrated best-in-class potential in patients with IgA nephropathy (IgAN) currently in Phase 3. The IgAN has no approved therapies that target the underlying cause, despite being the most common cause of primary (idiopathic) glomerulonephritis worldwide, affecting approximately 130,000 people in the U.S. Along the pipeline, the Alpine acquisition brought a great team of experts. Alpine stock performance from IPO to acquisition, source: TradingView. Due to accounting logics, Vertex continues to report some M&A transaction all over the FY2024. Hence you’ll notice that the company report a negative bottom line - i.e. net income. This can seem counterintuitive to an inattentive investor. In particular it is expected 2024 AIPR&D expenses of approximately $4.6 billion for the full year, of which $4.4 billion Alpine acquisition-related charge. With some of these costs related to compensation expense associated with cash-settled unvested Alpine equity awards. As per the classic definition, the “In-Process Research and Development” (IPR&D) comprises all intangible assets that are at the research or development stage. When acquired as part of a business combination (merger or acquisition), IPR&D are recognised and recorded at fair market value even if it is only at the research stage and not yet to be at the development stage. In the absence of business combination, IPR&D expenditures are expensed in the income statement but can be amortised if management can demonstrate that either alone, or in combination with other assets, the IPR&D is usable and will generate economic value into the future. Business valuers determine the value of IPR&D by applying appropriate methods for valuing intangible assets. What it all means is that all the R&D acquired right now from Alpine Immune Sciences ($4.4 billion) are expenses. No value will be extracted from that. As you may notice it is just an accounting problem. Not a real one, since the top line - i.e. product revenue - is healthily increasing. Even in the case of a failed M&A move, the cash was there for Vertex to deploy and the income loss is only temporary and not a sign of a structural problem. A case may be made about the management team, maybe in another article. Now that we’ve talked about past results, let’s try and see what the future holds. Let’s dive in the VX-548 compound. VX-548, or Suzetrigine, is a small-molecule for the treatment of moderate-to-severe acute pain. What’s more important is what this compound is not. It is not an opioid, hence no opioid-addiction. It’s the first of a potential new analgesic pharmacological strategy, with a novel pharmodynamics: selective small molecule inhibitors of NaV1.8 (a specific sodium channel). Yet, developing drugs for NaV1.8 is complex due to similarities across NaV channels (NaV1.1-1.9), but Vertex’s molecule is 30,000-fold more selective for NaV1.8, overcoming these challenges. That probably is the key difference from the Orion Corporation molecule (ODM-111) that was recently retracted from further development (“due to narrow therapeutic window of the molecule” cites the company). The molecule was only in Phase 1. Vertex has not only VX-548 in advanced Phase 3, but also another compound, VX-993. This next-gen selective NaV1.8 pain signal inhibitor is in Phase 2 study for an oral formulation for the treatment of moderate-to-severe acute pain following bunionectomy surgery. A Phase 1 trial, in the enrolling phase for dosage, is also underway for an intravenous formulation of the same compound. You will not be surprised so know that the FDA has granted Fast Track Designation to VX-993 in moderate-to-severe acute pain. But it happed for both its oral and intravenous formulations. VX-993 is a more potent follow-on to VX-548, with additional advantages, including suitability for IV formulations and potential combination use with NaV1.7. VRTX is making progress also in the NaV1.7 program, only in preclinical stage though. This could in the middle future complement NaV1.8 assets like VX-548 and VX-993. To sum up, the analgesic pipeline is very promising, yet not FDA-approved.
https://crsreports.congress.gov/product/pdf/R/R44576What is a PDUFA (Action) Date?


Financials - Balance Sheet and Income statement
A Slowing Company?


The Problem with AIPR&D = Acquired In-Process Research & Development expenses
Selected Pipeline
Suzetrigine, great clinical progression and a legal favorable environment
Other Painkiller Candidates, and More
On this novel line of treatment, we are optimistic because a U.S. nationwide survey underscores the urgent demand for non-opioid options, reflecting a significant opportunity to address the opioid crisis, which costs the U.S. $180 billion annually. This unmet need aligns with the commercial strategy for suzetrigine, including initiatives like nationwide retail distribution and financial assistance programs to facilitate patient access.
Furthermore, , such as the NOPAIN Act effective in January 2025 and the Alternatives to PAIN Act of 2022, is creating a supportive regulatory environment. These policies prioritize non-opioid options by removing barriers like step therapy requirements, ensuring equitable access, particularly for Medicare (older) patients. Together, these factors set the stage for suzetrigine to make a transformative impact in pain management. For some more context, the Alternatives to PAIN Act, passed by the Congress in 2022, would ensure that seniors have full access to non-opioid pain management options. Plus it allows patients and healthcare professionals to choose the treatment that is right for them. Under this bill, Americans on Medicare Part D, would never pay more for a non-opioid than they would for an opioid prescription. Meaning policy efforts like this aim to prevent barriers, such as requiring patients to use generic opioids before accessing suzetrigine. The main products remain linked to Cystic Fibrosis. KAFTRIO, the company’s blockbuster, hit another milestone. It is now approved and reimbursed in all EU countries, showcasing regulatory and reimbursement success. KATRIO is known as TRIKAFTA in the U.S., the brand name is different but the compound combination is the same: elexacaftor, tezacaftor, and ivacaftor. While the company strive to diversify from that, it also straighten its dominance in the market. The FDA accepted the New Drug Application (NDA) for Vanzacaftor/Tezacaftor/Deutivacaftor (Vanza Triple), a next-in-class combination therapy for Cystic Fibrosis (CF), granting a Priority Review with a PDUFA target action date of January 2, 2025. This treatment is aimed at CF patients aged 6 years and older who have at least one F508del mutation or another responsive CFTR mutation. The therapy is also validated by the European Medicines Agency (EMA), and regulatory submissions are ongoing in other regions. Despite the good news, the economic impact is expected to be modest, with a meaningfully lower single-digit royalty obligation compared to rate payable on Vertex’s current CF portfolio. All slides presented, are from official Earnings Call. Overall, the future seems to be rosy also thanks to solid management vision and ambitions. Indeed, VRTX management team remains on track for the “5-in-5 Ambition”, five new product launches over five years. They could be half way with the two expected launch early in 2025 (cystic fibrosis extension and new drug for acute moderate-to-severe pain). As you may already now from the previous article, the company pioneered the CRISPR space with the compound sold under the brand name CASGEVY. I will not dwell further into that. Just acknoledge that (i) it is therapy for sickle cell disease and beta thalassemia that is gaining momentum with patients, physicians, and policymakers; (ii) early launch is progressing well, with the first patient treated commercially and revenue recognized (in Q3-24 $2 million from the first patient dosed with CASGEVY was included); (iii) regulatory approval is expanding with approvals in Switzerland and Canada, and submissions planned for Kuwait and the UAE by year-end; All in all, CASGEVY is expected to become a multi-billion-dollar opportunity as adoption increases globally. Estimated to be around $0.3 billion for the U.S. alone - considering a 10% market penetration for the around $2 million treatment that can be beneficial to the 1’500 patients with transfusion-dependent beta thalassemia in the U.S. alone. Risks are related to: (i) expectations for Vertex’s continued growth in CF, including through new approvals and reimbursements for the treatment of younger patients, (ii) the beliefs regarding anticipated benefits of CASGEVY, expectations for increasing numbers of patients initiating cell collection, and expectations with respect to international access and reimbursement for CASGEVY, (iii) expectations regarding the potential benefits and commercial success of suzetrigine for the treatment of moderate-to-severe acute pain, including beliefs regarding the efficacy and safety of suzetrigine, and beliefs that suzetrigine has potential to provide effective pain relief without the limitations of opioids and other available medicines, (iv) expectations and status of the potential near-term commercial launch of the vanzacaftor triple, and plans to continue to advance new oral small molecule combination therapies for the treatment of CF, (v) expectations for the rest of the pipeline (VX-522 with share data in the first half of 2025; the SCD and TDT program; IV and oral formulation of VX-993; PNP patient populations; the continue development of NaV1.8 and NaV1.7 inhibitors for both acute pain and PNP; and other like but not limited to T1D programs with VX-264 initial data to be shared in 2025). Vertex, the Company The News: Suzetrigine's Incoming Action Date FDA Process and PDUFA Financials Acquired In-Process R&D (AIPR&D) Selected Pipeline Cystic Fibrosis Portfolio CRISPR Technology - Nobel-winning Tech in (Early) Action Expectations and Risks Disclaimer: I am not a financial advisor or registered investment advisor. The information contained in this article is for informational purposes only and should not be construed as financial advice. Past performance is not a guarantee of future results. You should always conduct your own research before making any investment decisions. Disclosure: I/we are long VRTX.
legislative momentumExpanding the Cystic Fibrosis Portfolio

Bonus: Nobel-winning Technology In (Early) Action
Expectations and Risks
TL;DR in 35 points